Almost everything comes with a label, from food to clothing to medicine to medical devices, and according to the FDA, face masks and cloth face coverings should come with labels too. More specifically, the Emergency Use Authorization for Face Masks released by the FDA April 24, 2020, includes labeling requirements for masks that are being made for the general public or for healthcare providers to contain the user’s respiratory secretions (i.e., coughs, sneezes, and other wet stuff sprayed from mouths and noses). In short, if you are making masks for other people in the context of the COVID-19 pandemic, whether they are cotton or nonwoven polypropylene (NWPP), you should include a face mask label with each mask that you sell or give away.
NOTA: This open source information is intended to help small-scale mask makers better meet the emergent demands of the COVID-19 crisis. Although the information and instructions provided below were thoroughly researched, developed with the aid of regulatory experts, and are believed to be accurate at the time of publication (May 7, 2020), they are not guaranteed to be complete or accurate. Compliance with all applicable laws and regulations is the sole responsibility of mask manufacturers. Check with local governing agencies for guidance and refer to the full April 24, 2020 Emergency Use Authorization.
Example Face Mask Labels
Two example labels designed to meet requirements of the FDA EUA are provided for makers/manufacturers of the MakerMask: Surge. The first is designed as a relatively simple single page User Label to be distributed with each mask for the general public. The second is designed for makers/manufacturers and includes an overview of responsibilities, as well as a more detailed Maker/Manufacturer Label. These labels will be updated as new and improved information becomes available:
User Labels (ver. 1.0): click the image below for the pdf of the MakerMask: Surge User Label (for the MakerMask: Fit User Label click here)
Maker/Manufacturer Label (ver. 1.0): click the image below for the pdf
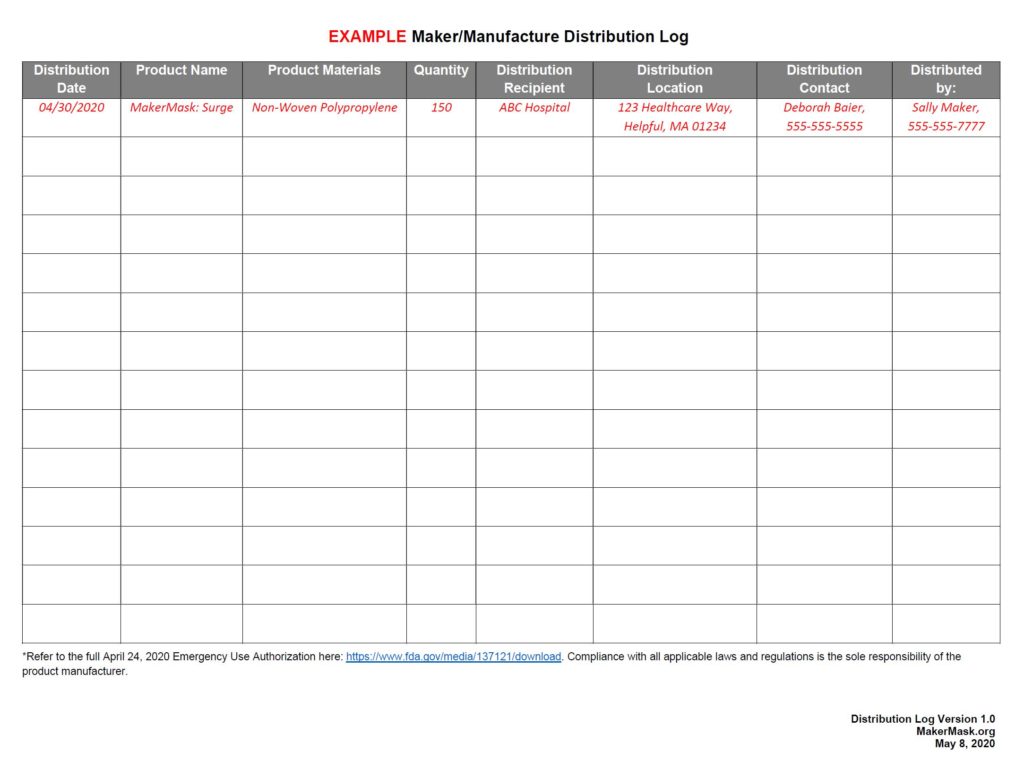
Example Mask Distribution Log
According to the new EUA, in addition to providing face mask label information, makers/manufacturers and mask distributors should keep track of to whom they distribute masks, and how many masks they’ve distributed.
Distribution Log (ver. 1.0): click the table below for the pdf
Minimum Labeling Requirements
For best practices for labeling, check with local governing agencies, refer to the full April 24, 2020 Emergency Use Authorization, and be sure to include the following information:
- Product Description. The product is labeled accurately to describe the product as a face mask.
- List of Materials. Include a list of the body contacting materials (i.e., the main body of the mask and the cloth ties).
- Recommended Cleaning and/or Disinfection Instructions. Instructions for recommended cleaning and/or disinfection materials and processes must be included.
- Warnings. This mask is NOT for use: 1) as a surgical mask, to provide liquid barrier protection; 2) in a clinical setting where the infection risk level through inhalation exposure is high; 3) for antimicrobial or antiviral protection or related uses or uses for infection prevention or reduction or related uses; 4) as a respiratory protective device; or 5) for high risk aerosol-generating procedures.
- Disclaimer. “The product has not been FDA cleared or approved. The product has been authorized by FDA under an EUA for use as source control by the general public as well as by HCP in healthcare settings to help prevent the spread of infection or illness during the COVID-19 pandemic. This product is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices, including alternative products used as medical devices, during the COVID-19 outbreak, under section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1) unless the authorization is terminated or revoked sooner.”
- Maker/Manufacturer Contact Information.
Download the open-source editable versions of documents referenced in this article:
- Example User Label
- Example Manufacturer Label
- Example Distribution Log
- Consolidated packet of all documents (.zip)
Update (May 12, 2020): The FDA just released (May 11, 2020) an overview with information and links to additional information about Face Masks for COVID-19, check it out at: https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/face-masks-and-surgical-masks-covid-19-manufacturing-purchasing-importing-and-donating-masks-during





5 pensamientos en “Label Me A Face Mask: FDA Labeling Requirements”
Comentarios cerrados.