
As a result of the COVID-19 pandemic, there are global shortages of N95 respirators resulting in large scale adoption of procedures and policies for the extended use and re-use of N95 respirators. In this post we do a deeper dive into the quantitative breathability data for the MakerMask: Cover, a breathable N95 mask cover. The MakerMask: Cover is designed as an N95 accessory to be worn on top of a NIOSH-approved N95. Used in this way: 1) it may help reduce surface contamination and soiling of the underlying N95 in cases of extended use or reuse and 2) it may be used as source control for N95s containing one or more exhalation valves.
A Breathable N95 Mask Cover
The MakerMask: Cover is constructed from a single layer of breathable, naturally water-resistant, mid-to-lightweight (≤90 gsm) spunbond nonwoven polypropylene (NWPP) and can be safely discarded after a single use or washed/disinfected for multiple uses. NOTE: Although the MakerMask: Cover may be used as a stand-alone face covering to cover the mouth and nose for source control, we recommend multi-layer designs such as the MakerMask: Surge or MakerMask: Fit for that purpose. The MakerMask: Cover is NOT intended for use as a surgical mask and is not FDA cleared or approved. Click the image below for the MakerMask: Cover User Label (pdf):
Breathability Data
Background
The American Medical Institute suggested that individual N95 respirators may be able to be used for a longer period of time by covering the respirator with a face mask (Sinkule et al, 2013). A recent paper from the Emergence Care Research Institute (ECRI) acknowledged that a homemade mask design could be used for this purpose, but stated that neither simple cotton fabrics nor paper masks would be appropriate due to the absorbent nature of the materials used in their construction (ECRI 2020). For any cover designed for use with an N95, the breathability of the combination is of tantamount importance. Previous work by Sinkule et al, 2013 (a NIOSH funded research project) established the functional breathability of fluid-resistant surgical masks when used in combination with N95s. Using similar methodologies, we worked with ATOR Labs to evaluate the breathability of water-resistant MakerMask: Covers used on top of N95s.
- Deeper Dive: Sinkule et al (2013) used automated breathing and metabolic simulators to investigate the potential effects of this combination on breathability (inhalation and exhalation resistance) as well as CO2 accumulation. They concluded that low levels of energy expenditure “would produce clinically small changes in inhaled breathing gases and breathing pressures resulting in a minimal effect on physical work performance.”
Results
The breathability of MakerMask:Covers when used with 3M N95 respirators was evaluated at ATOR Labs using ISO 16900 and NIOSH testing methodologies. Inhalation resistance, exhalation resistance, and carbon dioxide levels were evaluated (see Table 1). According to ATOR Labs, “All three mask coverings would pass NIOSH/NPPTL testing for form, fit and function” (see Appendix for Final Report).
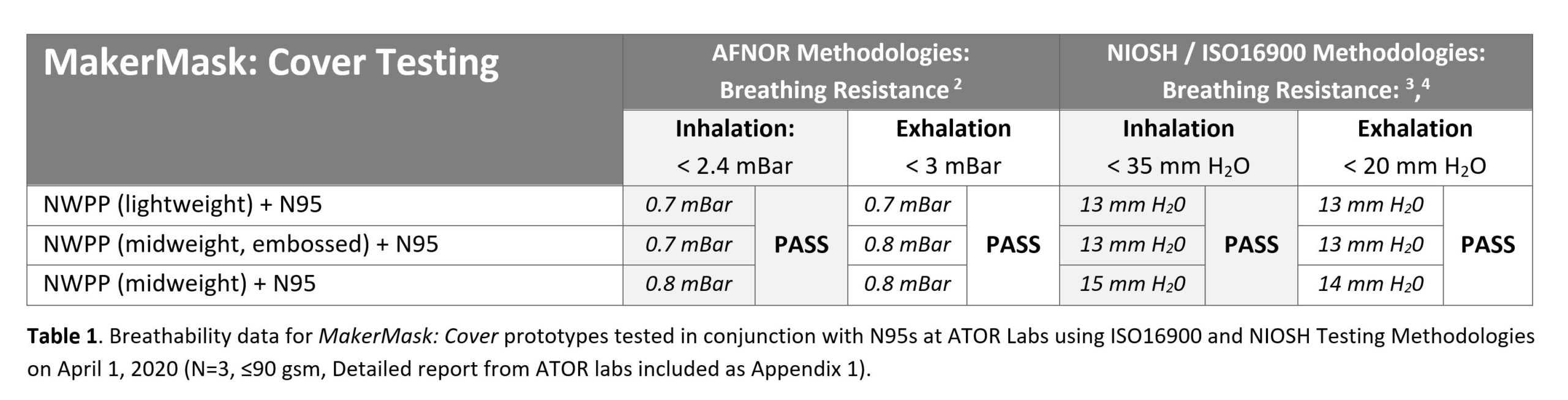
Conclusion
The breathability data presented here, and the material properties of nonwoven polypropylene (water resistance and suitability of the materials for washing/disinfection procedures) support the use of a nonwoven polypropylene MakerMask: Covers with N95s during the current COVID-19 public health crisis while global shortages of N95s and surgical masks persist. The MakerMask: Cover is a breathable N95 mask cover.

Appendix: ATOR Labs Final Report for the MakerMask: Cover

From: Rob Moran, Ator Labs
To: Jocelyn Songer, Ph.D
Subj: Soft mask over N95 mask
The soft masks were visually inspected prior to placement over a 3M N95 mask on the ISO head form. There were no visible tears, rips, or gross material deformities in the filter material or the mask covers. Masks are marked as JR1, JR2, and JR3.Testing was completed at 1542CDT on 24 April 2020. Testing was completed using ISO 16900 and NIOSH testing methodologies as adapted for use on the ATOR Labs Automated Breathing and Metabolic Simulator.Performance flow testing was performed using the ABMS set at a flow rate of 85 lpm in accordance with NIOSH TEB- APR – STP – 0003 DETERMINATION OF EXHALATION RESISTANCE TEST, AIR-PURIFYING RESPIRATORS STANDARD TESTING PROCEDURE. Results are reported in mmH2O: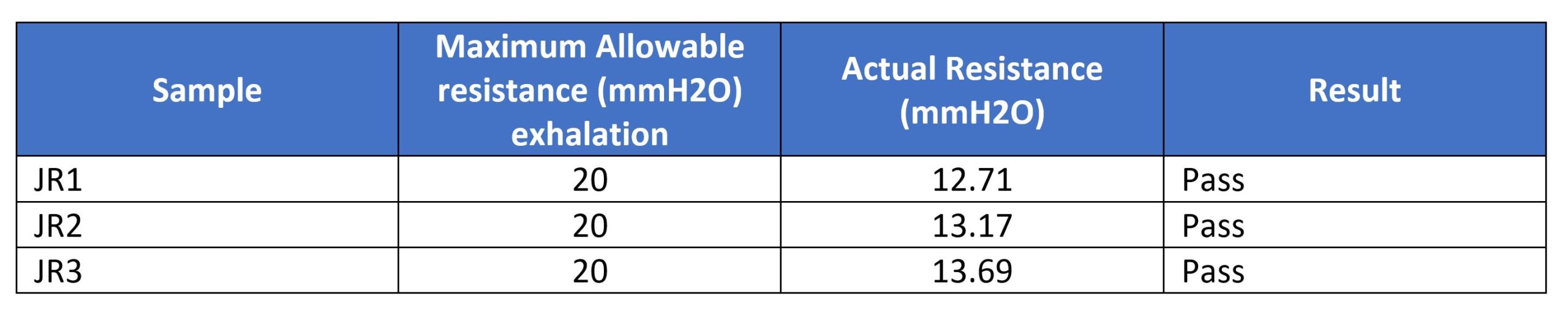 Performance flow testing was performed using the ABMS set at an instantaneous flow rate of 85 lpm in accordance with NIOSH TEB- APR – STP – 0007 DETERMINATION OF INHALATION RESISTANCE TEST, AIR-PURIFYING RESPIRATORS STANDARD TESTING PROCEDURE. Results are reported in mmH2O:
Performance flow testing was performed using the ABMS set at an instantaneous flow rate of 85 lpm in accordance with NIOSH TEB- APR – STP – 0007 DETERMINATION OF INHALATION RESISTANCE TEST, AIR-PURIFYING RESPIRATORS STANDARD TESTING PROCEDURE. Results are reported in mmH2O: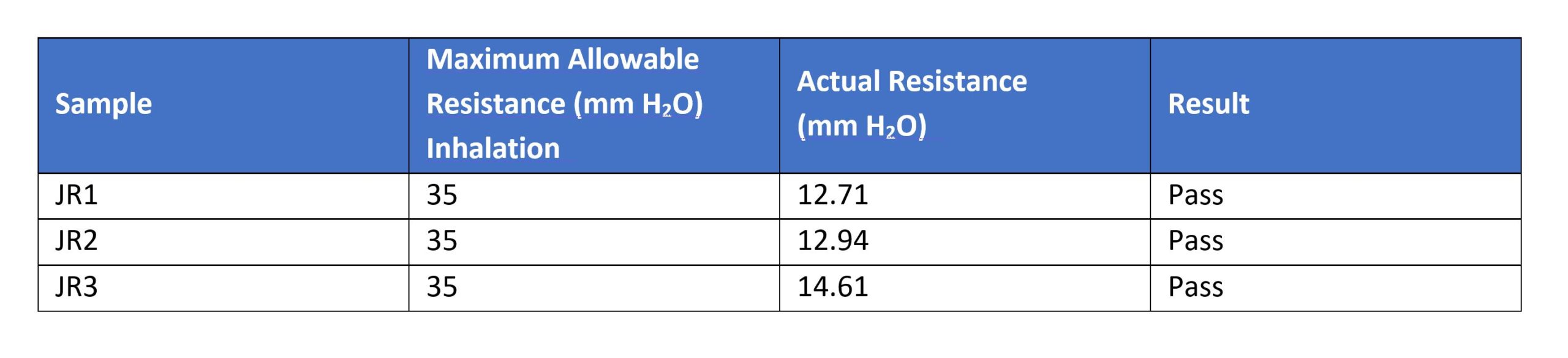 According to NIOSH “RCT-APR-STP-0064 DETERMINATION OF FACEPIECE CARBON-DIOXIDE AND OXYGEN CONCENTRATION LEVELS OF TIGHT FITTING, POWERED AIR PURIFYING RESPIRATORS WITH THE BLOWER UNIT OFF OR NON-POWERED RESPIRATORS STANDARD TESTING PROCEDURE”, a device’s averaged inhaled carbon dioxide level cannot exceed 2%.
According to NIOSH “RCT-APR-STP-0064 DETERMINATION OF FACEPIECE CARBON-DIOXIDE AND OXYGEN CONCENTRATION LEVELS OF TIGHT FITTING, POWERED AIR PURIFYING RESPIRATORS WITH THE BLOWER UNIT OFF OR NON-POWERED RESPIRATORS STANDARD TESTING PROCEDURE”, a device’s averaged inhaled carbon dioxide level cannot exceed 2%.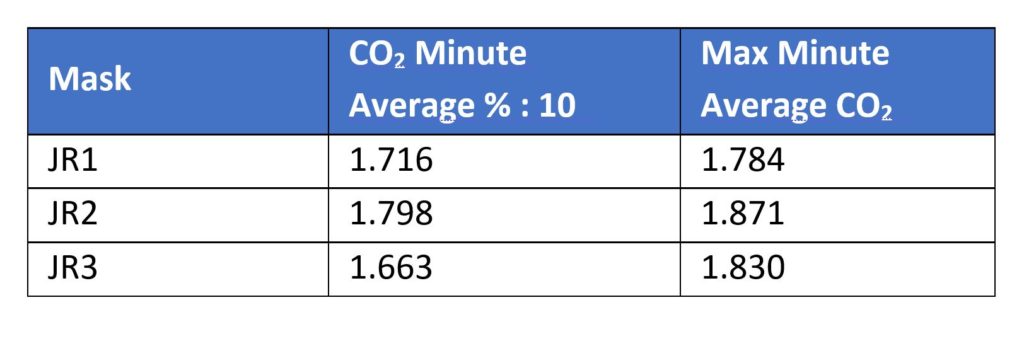 Conclusion: All three mask coverings would pass NIOSH/NPPTL testing for form, fit and function.
Conclusion: All three mask coverings would pass NIOSH/NPPTL testing for form, fit and function.







2 thoughts on “A Breathable Combination: An N95 plus a NWPP Mask Cover”
Comments are closed.